why we should care about
Pyrogen Testing
Learn about the various pyrogen testing methods available, the advantages & disadvantages of our solution, and decide if we can be a suitable partner for you.
What is pyrogen testing?
Definition of Pyrogens
If we want to do a pyrogen test, we must first clarify what we are looking for with our test methods.
Methods of Pyrogen Testing
We will introduce you to the methods commonly used at the moment.
Products that must be tested
This chapter will explain what kinds of products must be tested and which method is most suitable.
Regulatory bodies
Lastly, we will give you an overview of the legislation and explain which methods fulfill the criteria.
1. Definition of Pyrogens
What are Pyrogens?
Pyrogens are tiny particles that derive from viruses, bacteria, yeast, fungi, or chemical substances and can induce an inflammatory immune reaction when injected into the human body. A distinction is made between exogenous and endogenous pyrogens. While previously mentioned examples are categorized as exogenous pyrogens, the immune reaction does not directly result from those but endogenous pyrogens released by the immune system. Those endogenous pyrogens are cytokines, more specifically interleukins like Interleukin-6 (IL6), produced through exogenous pyrogens binding to their specific Toll-like-receptors (TLRs), expressed on the surface of monocytes. With this release of interleukins, the inflammatory cascade starts, resulting in a rise in body temperature in case of IL-6.
Exogenous Pyrogens
They derive from viruses, bacteria, etc., and bind to Toll-like-receptors (TLRs) on the surface of monocytes and thus induce an immune response.
Endogenous Pyrogens
Those are messengers of the immune system, e.g., interleukin 6 (IL-6), produced as a response to pyrogen detection by monocytes.

Sterile
Free from all living microorganisms such as bacteria or viruses that could reproduce in a human body.

Pyrogen free
Free from endotoxins and non-endotoxins that occur, e.g., when bacteria are killed through sterilization.
Endotoxins and Non-Endotoxins
With pyrogen testing, it is essential to consider the differences between endotoxin and non-endotoxin pyrogens. When a product is sterilized, for instance, by temperature increase, bacteria are killed and decomposed, but their fragments remain in the product.
fragments of gram-negative bacteria's cell membrane
Endotoxins
Fragments of gram-negative bacteria’s cell membranes are called endotoxins, while all other kinds of pyrogens are subsumed under the term non-endotoxin pyrogens (NEP). Endotoxins are also known as lipopolysaccharides (LPS), describing their structure of a sugar head and a lipid tail. The part in the LPS responsible for the toxicity of gram-negative bacteria occurring in the cell membrane is named lipid A. This lipid A then binds to the its specific TLR4 receptor on the monocytes and triggers an immune response.
all the other pyrogens
Non-Endotoxins
Examples of those non-endotoxin pyrogens are gram-positive bacteria, yeast, molds, and all the components of gram-negative bacteria except their cell membrane.
Some pyrogen tests can only recognize endotoxins, leaving all the non-endotoxins undetected.
Conclusion
We test our pharmaceutical products for exogenous pyrogens, which would cause the production of endogenous pyrogens when injected into the human body. Those exogenous pyrogens can be categorized either as endotoxin, deriving from the cell membrane of gram-negative bacteria, or as non-endotoxin pyrogens.
2. Methods for Pyrogen Testing
How to Test for Pyrogens?
There are four pyrogen testing methods on the market which are more or less commonly used. First, there is the Rabbit Pyrogen Test (RPT), the oldest one, which is about to be banned by the European Pharmacopoeia in 2026 and, therefore, increasingly less used. Second, the Limulus Amebocyte Lysate (LAL) test uses the horseshoe crab’s blood to detect endotoxins. The third one to mention is the Recombinant Factor C test (rFC) which uses the same mechanism as the LAL test, but for which the decisive component of the horseshoes’ crab blood is synthesized, and therefore no animal is used. Finally, there is the Monocyte Activation Test (MAT) which uses human monocytes to test for both sorts of pyrogens, endotoxin, and non-endotoxin.

Rabbit Pyrogen Test (RPT)
For a long time, the RPT was the only established and validated test method for pyrogen control. The technique is relatively easy; one puts a rabbit in a cage so it cannot move, injects whatever product one wants to test for pyrogens into his ear vein, and measures the body temperature. If the rabbit gets a fever, the product contains a significant amount of pyrogens; if there is no rise in body temperature, the product does not contain a substantial amount (for a rabbit) of pyrogens and is certified pyrogen-free.
Advantages
Firstly, this method has a few quite intuitive advantages. The test is easy to perform, you do not need highly trained personnel, and after approximately two hours, you get a result.
Disadvantages
The most prominent disadvantage is the use of approximately 400.000 rabbits per year. It is mandatory to use three animals which all have to show the same result; if one gets a fever and the other two do not, the test is invalid and has to be repeated. Therefore, the number of rabbits required for one pyrogen test is at least three but likely more, leading to high animal husbandry costs. Another issue is variability, which means your test results are not always reproducible. Additionally, just because the rabbit gets a fever does not mean that a human would react the same way. Consequently, the rabbit-test results are not always transferable to humans. One last disadvantage is that you cannot test all types of pharmaceuticals with the rabbit test. Due to the mechanism, the test cannot be applied to chemotherapeutic and immunosuppressive, and testing medical devices is quite complicated.
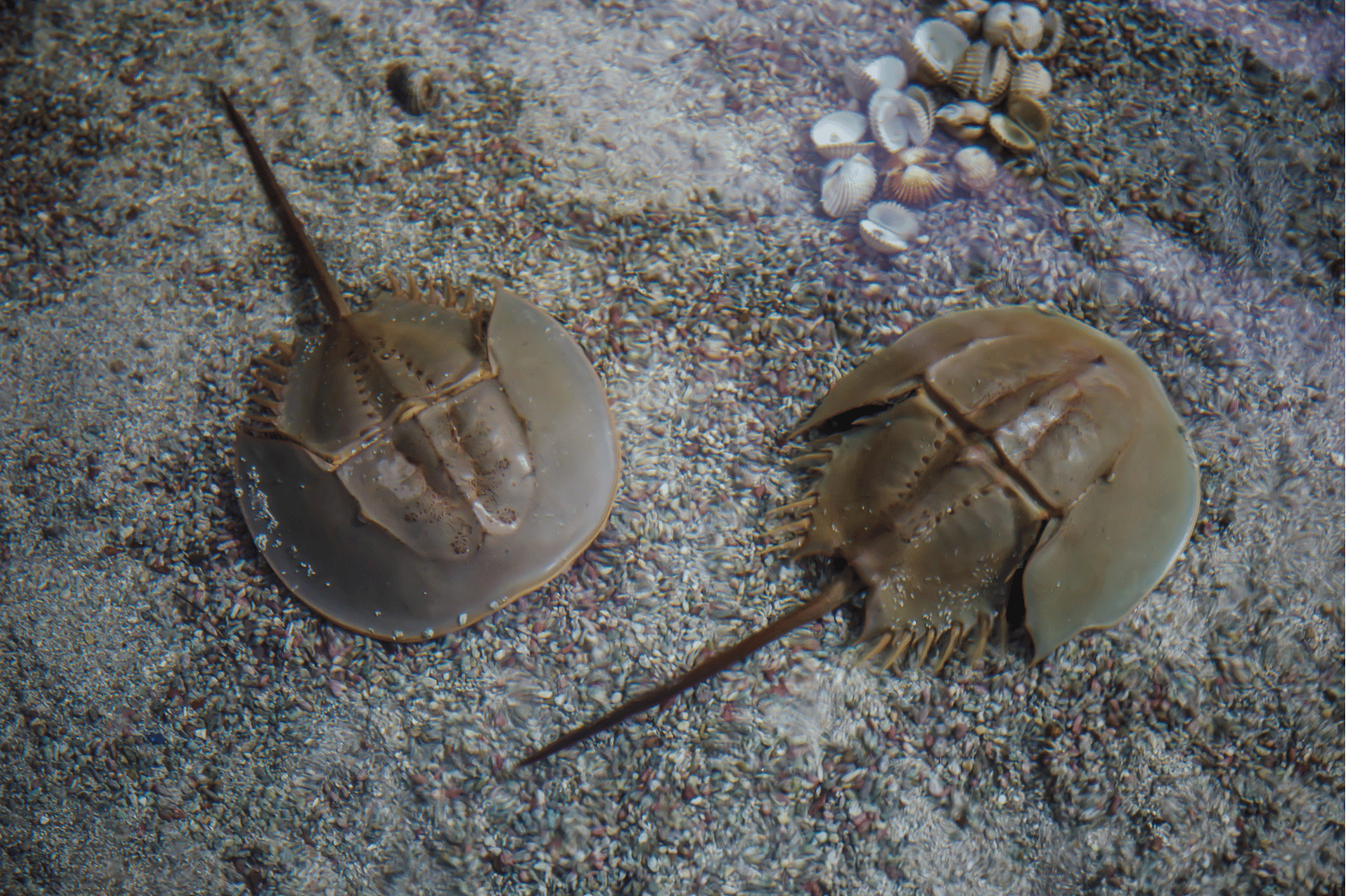
Limulus Amebocyte Lysat Test (LAL)
The LAL test is an enzymatic-based in vitro test using the horseshoe crab’s blood. The horseshoe crab’s blood contains a protein called Factor C (FC) which interacts with endotoxins. An addition of endotoxin to the blood leads to a reaction cascade, starting by FC, resulting in an enzymatic reaction in which a proclottting enzyme is activated and builds a gel clot. This gel clot is the marker for a positive test result and the presence of endotoxins in the tested substance.
Advantages
The first thing to mention is the simplicity of the test. One has to mix the blood with the test substance and get a result, a test for which no training at all is required. It is around 100 times more sensitive than the rabbit test. It can also be modified as a semi-quantitative test by adding the dye 4-Nitroaniline, which results in a coloring proportional to the endotoxin concentration.
Disadvantages
Even though the LAL test is performed as an in vitro test, it uses the blood of animals. This results in an estimated 50.000 horseshoe crabs killed per year for their blood to perform the LAL test only in the US.
But the disadvantage that is even more important is the incapability of this test to detect non-endotoxin pyrogens (NEP). As mentioned before, NEPs are responsible for about 40% of the detected pyrogen contaminations, but those NEPs cannot be recognized with the LAL test.
Moreover, probably due to the enzymatic mechanism, the LAL test is prone to interfere with the test sample and, therefore, is prone to the LER masking effect as well.
Recombinant Factor C Test (rFC)
This pyrogen test is similar to the LAL test because it uses an identical biological mechanism. The huge advantage of this method is that the recombinant Factor C is synthesized instead of utilizing the crab’s blood. Despite the animal use, the advantages and disadvantages are mostly the same as for the LAL test.
Monocyte Activation Test (MAT)
The MAT (EP 2.6.30 Monocyte Activation Test) is a cellular based assay to detect a broad range of pyrogens (both endotoxins and non-endotoxins) in pharmaceutical products (e.g. vaccines, monoclonal antibodies, hormone preparations). It uses human immune cells and measures the immune reaction to pyrogenic contaminations.
Advantages
The advantages of the MAT Test, in general, are the Human specificity, the low Lower Detection Limit (LoD) (1), the broad range of pyrogens that can be detected (2), and the semi-quantitative analysis of pyrogen contaminations (3). The MAT is suitable for testing all kinds of pharmaceutical products (4). Medical devices can be incubated directly with the immune cells. Therefore, even surface pyrogens are detectable.
There are also indications that the MAT is a good option to overcome low endotoxin recovery (LER) effects through diluting out interferences. That is possible due to the Lower Limit of Detection of 0.004 to 0.008 EU/ml.
Disadvantages
Nonetheless, the MAT has its disadvantages. The most severe obstacle is the complexity of a MAT assay. The quality of the test results depends on a wide range of factors. First, there are the immune cells and their activity, then there are various medium supplements that can lead to different results; moreover, there is donor variation within the immune cells, and lastly, it also depends on how the test is performed. There is a lot that can go wrong. Therefore, highly trained personnel is needed to perform the MAT, or the pyrogen testing must be sourced out.
Conclusion
Considering the fact, that the Monocyte Activation Test is the only test available on the market, covering all of the critical pyrogens, we come to the conclusion that the MAT is the future test method when it comes to pyrogen testing. To many of the disadvantages that we mentioned, we can provide you with a suitable solution.
3. Products that must be tested
What products must be tested for pyrogens?
According to general chapter 2.6.8. of the Ph. Eur., every parental drug batch must undergo pyrogen testing before release. Moreover, for every product, the MAT has to be proven as a suitable test method, causing no interferences with the product. To do that, there are product-specific validation tests required.
Batch release
testing
Product validation testing
4. Regulatory Bodies
What do Regulatory Bodies recommend?
The European Pharmacopoeia recommends in-vitro testing instead of in-vivo testing wherever possible without compromising patient safety. Since the MAT is a suitable replacement for the RPT, the Ph. Eur. is transitioning out of RPT until 2026, recommending the MAT as a replacement.
However, in the United States, until today, the Food and Drug Administration FDA still prefers the old-fashioned rabbit pyrogen test, which makes using MAT in the US infeasible. But there is hope that the FDA will follow the Ph. Eur. after its pioneer step of transitioning out the RPT.

