Monocyte Activation Test
Introduction to the MAT Research Monocyte Activation Test (MAT). Learn everything about our MAT technology and our approach to pyrogen testing.
Overview
Performing a MAT
We explain to you our best practices for performing a MAT to get the most out of our MAT Kit.
Cell Sources
There is currently a considerable discussion on which type of cells is the best for a MAT assay.
Reproducibility
One of the main questions regarding the MAT is whether the results vary too much to be comparable.
Discussions
Look into the discussion about the Limit of Detection, or Low Endotoxin Recovery effect.
1. Performing a MAT
How does our MAT work?
CD14+ monocytes are a subset of immune cells of the human body. These monocytes are professional pyrogen “detectors” and are most important for the human febrile fever response. A group of cell surface proteins, so-called Toll-Like Receptors, are pattern recognition receptors (PRRs) widely expressed on healthy monocytes and activate the immune reaction upon recognition of pyrogenic contamination to these receptors. The monocyte activation test (MAT) mimics this fever reaction “in a dish.”
The three steps to your pyrogen test

Immunological Reaction
The test sample is incubated overnight with human monocytes in a cell culture medium. The monocytes start to produce IL-6 if any pyrogenic contamination is present in the test sample.
IL-6 Secretion Analysis
The accumulation of secreted IL-6 by the monocytes in the cell culture medium can be quantified by a technique called “enzyme-linked immunosorbent assay” (ELISA). For the test samples as well as reference samples, the total IL-6 secretion is quantified.
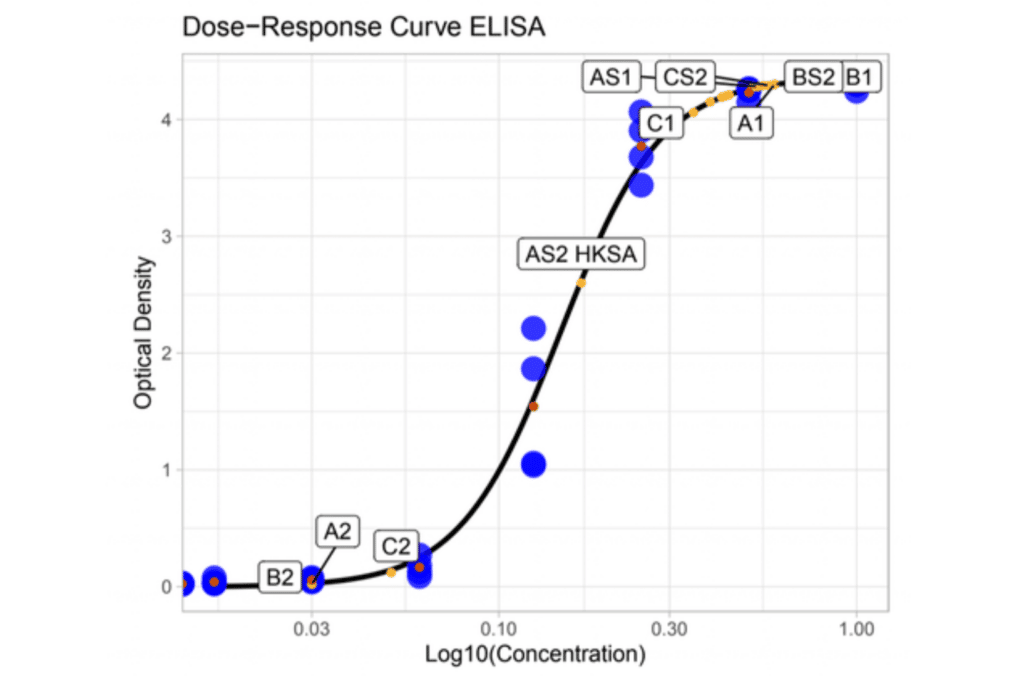
Quantifying the Pyrogenic Contamination
By interpolating the values found in the test sample to a known reference concentration (LPS), the endotoxin equivalent concentration (EEU/mL) can be calculated. If the calculated EEU/mL exceeds the contamination threshold, the sample can be considered to have pyrogenic contamination and needs to be disqualified for release.
2. Cell Sources
What are the different cells used for a MAT?
When thinking of a type of cell for the MAT, the question arises of which cells are more precisely detecting pyrogens and simultaneously most robust. So the first criterion for selecting a cell source is robustness. We want the cells to be resilient so that they are not harmed by environmental changes while transporting, centrifuging, cryopreserving, and all the other steps of the MAT. The second criterion is the sensitivity of the cells and the result’s reproducibility. The variation between different donors is a curse and a blessing at once: every donor has their own cytokine “profile.” Therefore every donor’s susceptibility varies for different TLRs; check out the section on donor pooling for more detailed information. Moreover, in order to be as sensitive as possible and coincidently deliver reliable results the cells must show a stable expression of TLRs.
Consequently, the second criterion concerns the equilibrium of sensitivity and the results’ reproducibility since the variation between donors increases the sensitivity while decreasing the reproducibility. Which of the two properties is favored depends on the test’s purpose (check out donor pooling to learn more). By this time, two different cellular platforms were established: primary immune cells from Peripheral Blood Mononuclear Cells (PBMC) and a cancerous monocyte cell line Mono-Mac-6 (MM6).
Comparison: MM6 vs. PBMC
MM6
MM6 cells are extracted from the blood of a leukemia patient. Thus, they are cancer cells, which are dividing fast. Therefore, one could think that this cell source is more robust than the PBMC, but dividing cancer cells are difficult to control. Hence mutations in this cell line have to be ruled out before use.
The second criterion, the sensitivity and reproducibility, is not met either, since the MM6 cells are known for a variety in TLR expression, which results in varying test results, especially regarding NEP detection. Therefore the European Pharmacopoeia explicitly mentions that MM6-based MAT assays are unsuitable for NEP detection.
PBMC
PBMC is a heterogeneous mixture of white human blood cells rather than a single cell source. The cells are extracted from the whole blood and cryopreserved at -180°C for transportation. At MAT Research, this is done in our own production facility in Besancon, France. Those cells cannot be cultured, so for every MAT is, a new vial of PBMC required. This results in obstacles regarding the robustness of the cells since they have to be frozen and thawed after shipping. Through our R&D, we established logistic procedures that minimize losses through transportation and freezing.
Coming to sensitivity, the PBMC has the advantage compared to MM6. They show a stable expression of TLRs, resulting in high reproducibility. Moreover, human immune cells are better at predicting the reaction of the human immune response to pyrogens than MM6, so the selectivity and transferability to the actual immune response is better with PBMC.
Conclusion
From our perspective, the PBMC is the more suitable cell source for Monocyte Activation Tests. The higher sensitivity and the better representation of the human immune response outweigh the disadvantage of the reduced robustness.
The European Pharmacopoeia (2.6.30) draws the same conclusion labeling the MM6-based MAT kit as “limited” for non-endotoxin pyrogen detection, whereas the PBMC-based MAT kits are classified as suitable for both, endotoxin and non-endotoxin detection.
How can one reach high reproducibility with MAT?
3. Reproducibility
Whether using single donor PBMC or pooled PBMC is an ongoing discussion in the field of Monocyte Activation Tests. That is why MAT Research provides you with both, depending on your needs and demands. Nonetheless we want you to be informed about the possible advantages and disadvantages regarding pooled or single donors.
Single Donor PBMC
When performing a pyrogen test with single donors, the MAT is conducted for a variety of single donors, e.g., four. That means carrying out the MAT four times to get a test result, thus resulting in a high effort. But this method’s advantage is that each donor’s data is collected individually. Therefore, varying results occur for different donors due to their individual cytokine profiles. Every human has different genetics resulting in another immune system that reacts individually to pyrogen contamination.
Thus, there is no such thing as sensitivity for “pyrogens,” but for a specific pyrogen. Pyrogens bind to their particular TLR, and donor variations result from different quantities of those TLRs. For instance, when one of the four donors expresses more TLR2s, he is more sensitive to a pyrogen in your test sample. Those specific immune responses can be revealed if the donors are tested individually. If, however, pooled PBMC is used, those differences between donors would average and not occur in the test result. Therefore, single-donor PBMC’s combined sensitivity is much higher than the pool’s, as the individual differences are highlighted in the first mentioned and averaged out in the latter.
Pooled Donor PBMC
For the sake of comparison, four pooled donor PBMC will be used as a proxy for any form of pooling. While four MAT assays have to be executed to perform one pyrogen test with single donors, the amount of assays is reduced to one when testing with pooled PBMC. In addition, through pooling the PBMC, the donor variations balance out, leading to more consistent test results. Let’s apply this to an example. Testing a substance for pyrogens with four single donors delivers three negative and one positive outcome. However, if this substance were tested with pooled PBMC, the result would be just negative, not detecting that some patients could develop an inflammatory response to the tested substance.
While this has to be perceived as a risk in terms of patient safety, an advantage of the procedure is the larger batch size. Using four donors pooled PBMC means four times increased batch size and thus less effort in adopting practices to batch-specific changes.
Conclusion
Despite the higher expenditures, at MAT Research, product-specific validation tests are always done with single donor PBMCs to ensure the highest accuracy in the test results. Therefore, we can surely reveal interferences due to our heightened sensitivity. When we do routine testing, we use pooled donors to shift the balance of sensitivity vs. reproducibility more to the latter.
The legal regulations allow both single-donor testing as well as pooled donor testing. Though, if there is one positive test within single donor testing, the test has to be repeated with four donors. If then all are tested negative, the batch can be released. Pooled donor testing has the advantage for the manufacturer that, e.g., this one positive test is balanced out.
What impact have LoD, LER & co.?
4. Discussions
Monocyte Activation Tests are still in development. Not many articles explain the importance of the Limit of Detection or the Low Endotoxin Recovery effect; thus, we want to give you an overview of these terms.
Limit of Detection
The MVD (maximum validated dilution) can be calculated by dividing the CLC (contaminant limit concentration) by the LoD (limit of detection). This is an important parameter of the monocyte activation test when pharmaceutical products (samples) need extensive dilution (high MVD) to overcome the possible interference with the test or products with a low contaminant limit concentration. Monocyte activation tests with a low LoD value increase the MVD and therefore broadens the range of pharmaceuticals that can be tested.
So how important is the LoD, and how is it calculated?
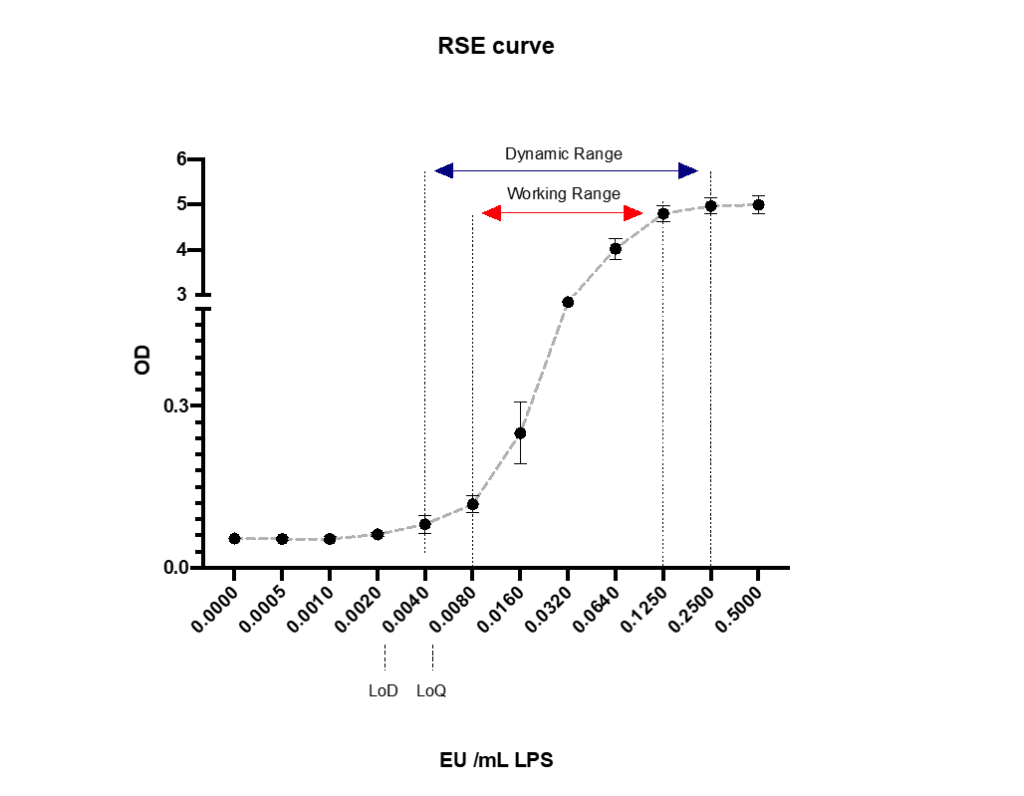
First, let’s discuss the different ranges that can be found in the monocyte activation test. Like every analytical assay, there are different detectable ranges to be found. In the monocyte activation test, we can determine the following ranges (figure 1). By now, the term LoD has been changed to “assay sensitivity” by the European Pharmacopoeia.
Dynamic range:
The Dynamic range are the concentrations between the minimal (LoD) and maximum detectable value; however, a non-linear dose response. It is the largest range of the analytical assay, but with high uncertainty and unacceptable to reliably analyze your data. Using this range to a minimal extent bears the risk of measuring the statistical beta error, resulting in false positive test outcomes.
Working range:
The working range are the concentrations between the first reliably detectable value (LoQ) up to the maximum response. This range is mostly used in analytical assays, like the monocyte activation test (MAT), to detect pyrogens in pharmaceuticals.
Endotoxin Masking Effects
The endotoxin recovery is measured to exclude interferences between the MAT and the product that is supposed to be tested. The idea is to eliminate the potential risk of contamination with masked pyrogens that are inactive and thus not detectable but turn active again when injected into a human body. Therefore a product sample is spiked with a certain amount of endotoxin. After that, the endotoxin quantity is measured with a MAT. If the measured quantity is within the range of 50 to 200% of the added amount of endotoxin, the MAT is validated for this product.
The endotoxin masking effect describes when the measured endotoxin quantity is below 50% of the added amount. This can be due to interferences between the monocytes and the test sample, resulting in no IL-6 secretion, or the test substance binding to IL-6, making it undetectable with the ELISA. In the end, one does not exactly know how those interferences arise, but they might falsify the test outcome. Since the added quantity of pyrogens could not be correctly measured, pyrogens already present in the product could also interfere. Therefore the batch cannot be tested on pyrogen contamination and is not released.
One option to overcome this masking effect is diluting the test sample to the extent that it still lies above the Maximum Valid Dilution (MVD) and hoping that the interferences do not occur. Since the MVD is defined as CLC / LoD, a lower LoD will automatically lead to a higher MVD and increase the chance that the interferences can be diluted out. But using the LoD as a reference to calculate the MVD is problematic because of the increased likelihood of false positive test results due to the measurement of the beta error. Therefore, instead of the LoD, we recommend using the first reliably detectable value (LoQ) to minimize the possibility of false positive tests.
Nonetheless, using the LoQ, the MAT still has the highest sensitivity and is, therefore, the most suitable test technology if you struggle with masking effects.
Low Endotoxin Recovery Effect
First, the Low Endotoxin Recovery Effect is still an ongoing discussion, and the clinical significance is not yet proven.
The LER effect is a subform of the endotoxin masking effects and occurs when an undiluted sample is spiked with endotoxin, but the endotoxin cannot be recovered. This phenomenon continues to show up when the sample is diluted but does not show up when it is diluted first and then spiked. This raised concerns that the masking continues when the product is diluted, bearing the risk of inactive, masked pyrogens contained in the product that could turn active again when injected into the human body. The LER effect was discovered many times using the LAL test, but there is still too little data available for the MAT assay to make a scientific statement. Until today, the LER effect has not been explored well enough, neither to say if it poses a threat to patient safety nor to establish protocols to avoid the occurrence.